Answer: The molarity of Iron (III) chloride is 0.622 M.
Step-by-step explanation:
Molarity is defined as the number of moles present in one liter of solution. The equation used to calculate molarity of the solution is:
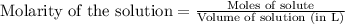
Or,
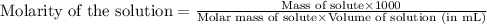
We are given:
Mass of iron (III) chloride = 1.01 g
Molar mass of iron (III) chloride = 162.2 g/mol
Volume of the solution = 10 mL
Putting values in above equation, we get:
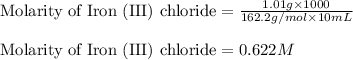
Hence, the molarity of Iron (III) chloride is 0.622 M.