Answer:
C)
Step-by-step explanation:
The first step is to write the whole reactions:
A)
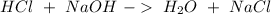
B)

In A) reaction we will have an acid-base reaction (double replacement reaction). In this reaction, the oxidation number is the same for all atoms on both sides of the reaction.
In B) the oxidation number of Cu will change from zero to +2 and the oxidation number of Ag will change from +1 to zero. So, the Cu will be oxidized and the Ag will reduce. So, if we have a redox reaction for B) the charge must be equal on both sides.