Hello!
The answer is:
The correct option is option C.
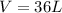
Why?
To calculate the volume of the gas sample, we need to use the Ideal Gas Law, this equation is used to relate the pressure, the volume, the mass and the temperature of a gas, so, we can isolate the volume from it.
The Ideal Gas Law equation is equal to:

Where,
P is the pressure (in atm)
V is the volume (in liter)
n is the mass of the gass (in mole)
T is the temperature of the gass (absolute temperature)
So, from the statement we know that:
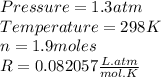
Then, substituting and calculating we have:

Now, rounding to the nearest whol number we have that the volume is equal to 36 L.
Hence, the correct option is option C.
Have a nice day!