Answer:
The percentage of the student is 58.17%.
Step-by-step explanation:
Expected yield of aluminum oxide = 115.2 g
Actual yield of aluminum oxide produced =66.9 g
The percentage yield is calculated by dividing actual yield by expected yield and then multiplying it with hundred.
Percentage yield:
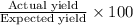
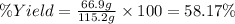
The percentage of the student is 58.17%.