Answer: The correct answer is Option B.
Step-by-step explanation:
Precipitate is defined as the insoluble solid substance which is formed when two different aqueous solutions are mixed. It settles down at the bottom of the solution.
For the given chemical equation:
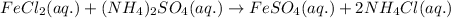
The products formed in the reaction are ferrous sulfate and ammonium chloride. Both the products are soluble in aqueous solutions. Thus, no precipitate will be formed in the reaction.
Hence, the correct answer is Option B.