Answer:
U
Step-by-step explanation:
The first law of thermodynamics states that:
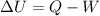
where
U is the internal energy of the gas, which represents the sum of the chemical and thermal energy stored in the atoms and molecules of the gas
 represents the variation of internal energy
represents the variation of internal energy
Q is the heat absorbed by the system
W is the work done by the system
So, the sum of the chemical and thermal energy stored in atoms and molecules is represented by U, the internal energy.