Answer:
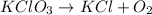
(C) is correct option
Step-by-step explanation:
Balanced equation :
In balanced equation, The number of atoms of each element in product side equal to the number of atoms of each element in reactant side.
The equation is not balanced in option C.
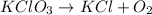
In this equation, The number of oxygen element in product side is not equal to the number of oxygen element in reactant side.
All equations are balanced.