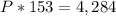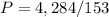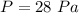Answer:
Option D.) 28 Pa
Explanation:
we know that
A relationship between two variables, x, and y, represent an inverse variation if it can be expressed in the form
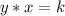 or
or
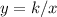
In this problem
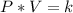
step 1
Find the value of k
For
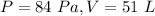
substitute and solve for k
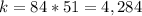
step 2
If the volume is expanded to 153 L, what will the new pressure be?
we have
The equation is equal to
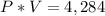
For
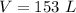
substitute