Hello!
The answer is:
The percent yield of the reaction is 32.45%
Why?
To calculate the percent yield, we have to consider the theoretical yield and the actual yield. The theoretical yield as its name says is the yield expected, however, many times the difference between the theoretical yield and the actual yield is notorious.
We are given that:
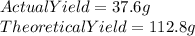
Now, to calculate the percent yield, we need to divide the actual yield by the theoretical and multiply it by 100.
So, calculating we have:
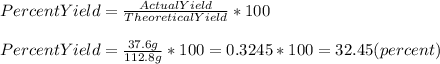
Hence, we have that the percent yield of the reaction is 32.45%.
Have a nice day!