Answer: Option (E) is the correct answer.
Step-by-step explanation:
Electronegativity is defined as the ability of an atom to attract electrons towards itself while formation of bond.
For example, chlorine is electronegative in nature and hence it will readily combine with potassium atom as potassium has one extra electron in its valence shell.
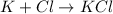
Thus, we can conclude that electronegativity of an atom is its ability to attract electrons during bond formation.