Answer:
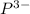 Anion Gained 3 18
Anion Gained 3 18
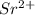 Cation Lost 2 36
Cation Lost 2 36
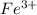 Cation Lost 3 23
Cation Lost 3 23
Step-by-step explanation:
Looking at the ionic notation, the negative symbol (-) indicates that there are more electrons than protons and the positive symbol (+) indicates that there are more protons than electrons.
The number tells you how many was gained or lost.
To determine how many electrons are left, you base this off how many protons there are. The number of protons in an atom is expressed by the atomic number. In a stable atom, you have an equal number of protons and electrons.
Ions occur when electrons are gained or lost.
A cation is positively charged because it LOST an electron. Since there are more protons than electrons, the charge would be positive.
An anion is negatively charged because it gained an electron. Since there are more electrons than protons, the charge would be negative.
Using the explanation above, you can see how the answers were obtained. As for the last column, just use basic math to do this.
The atomic number of Phosphorus (P) is 15, so this means that there are 15 protons. Since it gained 3 electrons, just add 3 to 15:
15 + 3 = 18
Sr has an atomic number 38. Since it lost 2 electrons, just subtract 2 from the atomic number.
38-2 = 36
Fe has an atomic number 26. It lost 3 electrons, so we subtract again.
26-3 = 23