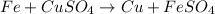Answer: Single replacement
Step-by-step explanation:
Single replacement reaction is a chemical reaction in which more reactive element displaces the less reactive element from its salt solution.
Iron being more reactive than copper would displace copper from its salt solution and thus will generate copper and iron sulphate.
The chemical equation representing iron metal plus copper (II) sulfate solution yields iron (II) sulfate solution and copper metal is: