Answer: The correct answer is they have same number of particles.
Step-by-step explanation:
According to mole concept:
1 mole of an element or compound contains
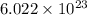 number of particles.
number of particles.
The number is known as Avogadro's number.
We are given:
One mole of uranium element and 1 mole of carbon dioxide molecule.
These two substances have same number of particles equal to Avogadro's number.
Hence, the correct answer is they have same number of particles.