Answer:
Lets Write Down the Given Initial Conditions.
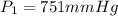

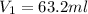
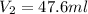
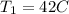
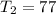
In Order to Solve for the Unknown:

we must use the Ideal Gas Law to Solve for the Second Unknown pressure:
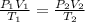
Then Rearrange this equation in a form where P2 can be solved from:
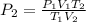
Then Insert the Values from above to solve:
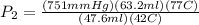
The Answer is : 1830 mmHg considering sig figs