Answer:
Lithium
Step-by-step explanation:
The equation for the photoelectric effect is
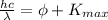
where
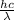 is the energy of the incident photon, with
is the energy of the incident photon, with
h being the Planck constant
c is the speed of light
 is the wavelength of the photon
is the wavelength of the photon
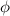 is the work function of the metal (the minimum energy needed to extract the photoelectron from the metal)
is the work function of the metal (the minimum energy needed to extract the photoelectron from the metal)
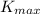 is the maximum kinetic energy of the emitted photoelectrons
is the maximum kinetic energy of the emitted photoelectrons
In this problem, we have
 is the wavelength of the incident photon
is the wavelength of the incident photon
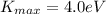 is the maximum kinetic energy of the electrons
is the maximum kinetic energy of the electrons
First of all we can find the energy of the incident photon
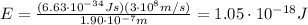
Converting into electronvolts,

So now we can re-arrange the equation of the photoelectric effect to find the work function of the metal
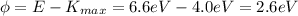
So the metal is most likely Lithium, which has a work function of 2.5 eV.