Answer:
The De Broglie wavelength decreases
Step-by-step explanation:
The relationship between the De Broglie wavelength of a particle and its momentum is given by
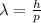
where
 is the De Broglie wavelength of the particle
is the De Broglie wavelength of the particle
h is the Planck constant
p is the momentum of the particle
As we see from the formula, there is an inverse relationship between the De Broglie's wavelength and the momentum. Therefore, we can conclude that:
- if the momentum of the electron increases,
- its De Broglie wavelength will decrease
and vice-versa.