Each element or compound has a molar mass, which is calculated by multiplying the atomic mass of each element by the amount of atoms of that element, and summing the results of each element. The molar mass is measured in g/mol. So you divide the mass in grams by the molar mass to get the amount of moles.
Example:
There are 5g of water.
Calculate the amount of moles.
The water's formula is H2O, so the molar mass of it is
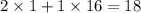
g/mol.
The amount of moles is:
5g ÷ 18g/mol ~ 0.28mol