Answer:
B. It does work or increases thermal energy.
Step-by-step explanation:
As we know by first law of thermodynamics that
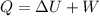
now we have
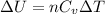
so internal energy is based on the change in temperature of the gas
Q = heat given to the system
W = work done by the gas
now we know that when heat is given to the system then by energy conservation work some of its heat is used to increase the temperature of gas as its internal energy and other part of heat is used to do some work.
so correct answer will be
B. It does work or increases thermal energy.