Answer:
Option a
Step-by-step explanation:
a)
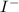
Atomic number of I = 53
No. of electrons = atomic number + no. of negative charge
= 53 + 1 = 54
Xe
Atomic number of Xe = 54
In a neutral atom, no. of electron is equal to no. of protons.
So. no. of electron in Xe = 54
a)
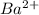
Atomic number of I = 56
No. of electrons = atomic number - no. of positive charge
= 56 - 2 = 54
All the species in the set a have 54 electrons.