Answer:
5.29 moles
Step-by-step explanation:
In order to calculate this you just have to divide the grams of the element that there are by the grams of that given atom that make uo for a mol, in this case for carbon 12.01 grams make up a mol, so we just have to divide the gram that we have by 12.01 grams:
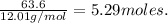
So we know that there are 5.29 moles in 63.6 grams of carbon.