Answer : The correct option is, the ratio of the volume and temperature in kelvins
Explanation :
According to the Charles' Law, the volume of gas is directly proportional to the temperature of the gas at constant pressure and number of moles.
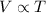 (At constant pressure and number of moles)
(At constant pressure and number of moles)
or,
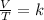
where,
V = volume of gas
T = temperature of gas
k = constant
As per question, when the pressure and number of particles of a gas are constant then the ratio of the volume and temperature in kelvins.
Hence, the correct option is, the ratio of the volume and temperature in kelvins