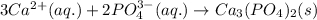Answer: The net ionic equation for the following precipitation reaction will be
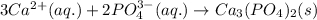
Step-by-step explanation:
Precipitation reaction is defined as the reaction in which two soluble salts combine in aqueous state to form one insoluble product known as a precipitate.
For the reaction of calcium chloride and potassium phosphate, the reaction follows:
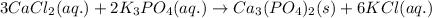
The ionic equation for the above reaction follows:
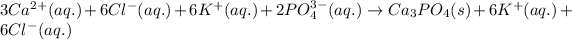
To write the net ionic equation, spectator ions are not written because these ions lie on both the sides of the reaction and does not affect equilibrium. These ions are ignored in writing the net ionic equation.
Hence, the net ionic equation becomes: