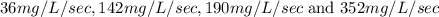Answer : The reaction rate at
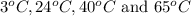 are
are
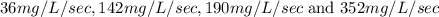
Solution : Given,
Mass of tablet = 1000 mg
Volume of water = 0.200 L
The given formula will be,
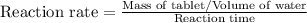
Now we have to calculate the reaction rate at different temperatures and reaction time.
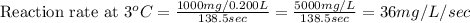
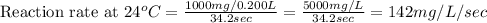
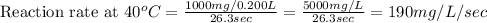
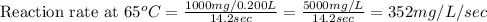
Therefore, the reaction rate at
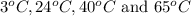 are
are