Answer: Option (C) is the correct answer.
Step-by-step explanation:
A hydroxide ion is an ion that has -1 charge and contains an oxygen and hydrogen atom together.
So, basically a hydroxide ion is
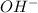 ion.
ion.
Where as a hydrogen ion is defined as the ion in which a hydrogen atom holds a positive charge, that is,
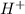 .
.
A proton is a sub-particle present inside the nucleus of an atom and it holds a positive charge. Symbol of proton is
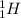 .
.
Thus, we can conclude that the statement they have a negative charge, is true of hydroxide ions.