Step-by-step explanation:
When the ice melts but there is no change in the temperature. The only phase change will occur in this case.
The heat energy is spent in changing the phase. The ice will get convert into water. On increasing the pressure, melting point increases.
When the solid water (ice) is slowly warmed from a low temperature. The minimum external pressure
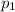 must be 610 Pa which is applied to the solid if a melting phase transition is to be observed.
must be 610 Pa which is applied to the solid if a melting phase transition is to be observed.