Step-by-step explanation:
Tin is the 50th element of the periodic table and has many isotopes. Three of its isotopes are given which are:
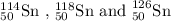
General representation of an isotope is
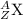
where,
A = atomic mass of the element
Z = atomic number of the element
X = symbol of the element
Atomic number is equal to the number of electrons or number of protons.
Atomic number = Number of electrons = Number of protons
Mass number is defined as the sum of number of neutrons and protons.
Mass number = number of protons + number of neutrons
- For Isotope
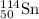
Atomic number = number of electrons = number of protons = 50
Mass number = 114
Number of neutrons = 114 - 50 = 64
- For Isotope
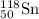
Atomic number = number of electrons = number of protons = 50
Mass number = 118
Number of neutrons = 118 - 50 = 68
- For Isotope
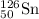
Atomic number = number of electrons = number of protons = 50
Mass number = 126
Number of neutrons = 126 - 50 = 76