Answer:- 330 L
Solution:- At constant pressure, volume is directly proportional to the kelvin temperature.
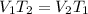
where,
 is the initial volume at initial temperature
is the initial volume at initial temperature
 and
and
 is the new volume at temperature
is the new volume at temperature
 .
.
From given info,
 = 160 mL
= 160 mL
 = -125 + 273 = 148 K
= -125 + 273 = 148 K
 = 29.0 + 273 = 302 K
= 29.0 + 273 = 302 K
 = ?
= ?
Let's plug in the values and solve this for new volume.
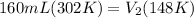
On rearrangement:
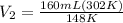
[tex]V_2] = 326 L
If we round the answer for two sig figs(as given in initial volume) then it will be 330 L.
So, the new volume of the balloon is 330 L.