Step-by-step explanation:
An ammonia
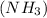 molecule contains electronegative nitrogen atom. The difference between electronegativity of nitrogen and hydrogen atom is high.
molecule contains electronegative nitrogen atom. The difference between electronegativity of nitrogen and hydrogen atom is high.
Therefore, nitrogen will attract the shared pair of electrons more towards itself. As a result, there will be partial positive charge on hydrogen and partial negative charge on nitrogen.
Thus, due to these charges
 is polar in nature.
is polar in nature.