Step-by-step explanation:
It is known that in 1 mole there are
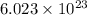 atoms or molecules.
atoms or molecules.
It is given that there are
 carbon molecules. Therefore, calculate the moles as follows.
carbon molecules. Therefore, calculate the moles as follows.
Number of moles =
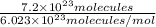
= 1.196 mol
Thus, we can conclude that there are 1.196 mol in
 carbon molecules.
carbon molecules.