Step-by-step explanation:
The given data is as follows.
No. of moles =
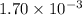 , V = ?
, V = ?
T = 20.2 + 273 K = 293.2 K, P =
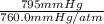 = 1.046 atm, R =
= 1.046 atm, R =
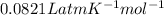
Calculate the volume using ideal gas equation as follows.
P V = n R T
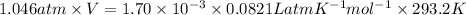
V =
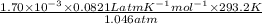
=
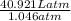
=
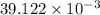 L
L
Thus, we can conclude that volume of the gas is
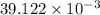 L.
L.