The reactions are
1)
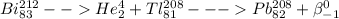
In this reaction the Bismuth gives an alpha particle and followed by beta decay
2)
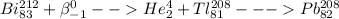
In this reaction the Bismuth is undergoing beta bombardment followed by alpha decay.
3)
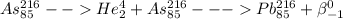
This is not a reaction of Bismuth.
4)
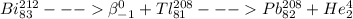
This is first beta elimination followed by alpha elimination.