Charles law gives the relationship between volume and temperature of a gas. It states that for a fixed mass of gas at constant pressure, volume is directly proportional to temperature in kelvin.
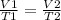
Where V1 and T1 are volume and temperature respectively for the first instance
and V2 and T2 are volume and temperature respectively for the second instance
temperature should be in the kelvin scale so °C have to be converted to Kelvin
T1 - 21.3 °C + 273 = 294.3 K
substituting the values in the above equation

T2 = 98.1 K
the final temperature in the flask is 98.1 K
in Celsius scale its - 98.1 - 273 = -174.9 °C
therefore final temperature is 98.1 K / -174.9 °C