Answer: There are
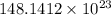 atoms of carbon in the given moles.
atoms of carbon in the given moles.
Step-by-step explanation:
We are given 24.6 moles of carbon and we need to find the number of atoms.
According to mole concept:
1 mole of an element contains
 number of atoms.
number of atoms.
So, 24.6 moles of carbon will contain
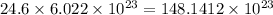 atoms.
atoms.
Hence, there are
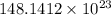 atoms of carbon in the 24.6 moles of carbon.
atoms of carbon in the 24.6 moles of carbon.