Answer: The NaI will be most dissociated in 0.1M
Step-by-step explanation:
NaI is an ionic compound having
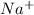 and
and
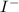 ions. It is also a strong electrolyte as it completely gets dissociated into its respective ions.
ions. It is also a strong electrolyte as it completely gets dissociated into its respective ions.
This behaves as a strong electrolyte when it is very dilute because when the solution is concentrated, the positive and negative charges will form ion pairs.
Hence, NaI will be most dissociated in the solution having 0.1M