here we have to choose the limiting reactant in the given unbalanced reaction.
The 5.6 moles of aluminum will be the limiting reactant in this reaction.
The reagent or reactant which is totally consume in a reaction is called the limiting reagent or reactant.
The unbalanced reaction is Al + H₂O → Al₂O₃ + H₂
On balancing 2Al + 3H₂O = Al₂O₃ + 3H₂
As we can see that 2 moles of aluminium (Al) reacts with 3 moles of water (H₂O). Thus 5.6 moles of Al reacts with
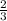 ×5.6 = 3.733 moles of water.
×5.6 = 3.733 moles of water.
Henceforth the 5.6 moles aluminum will be the limiting reactant as it is totally consume, but only 3.733 moles of water is reacting and the rest 6.2 - 3.733 = 2.467 moles of water will remain excess in this reaction.
Thus the aluminum will be the limiting reactant.