Answer: option 'A' is correct.
Explanation:
Since we have given that
Quantity of radioactive substance = 200 milligrams
Rate of decay = 25%
Number of weeks = w
As we know the formula for "Compound Interest ":
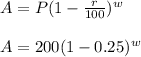
So, here , P=200 milligrams i.e. the initial amount
So, It is the product of the initial amount and the decay factor after w weeks.
Hence, option 'A' is correct.