Step-by-step explanation:
When an element is displaced by another element in a compound then it is known as a single displacement reaction.
For example,
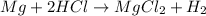 is a single displacement reaction.
is a single displacement reaction.
Similarly when a hockey player has the puck in the corner of the rink. An opposing player muscles him aside and emerge with the puck displays that a single player is displacing a person.
Thus, we can conclude that this situation is most analogous to single displacement reaction.