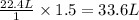Answer: a) 90.5g
b) 33.6 L
Explanation:-
Molar mass of tyrosine
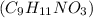 = 181 g/mol
= 181 g/mol
According to Avogadro's law, 1 mole of every substance weighs equal to its molar mass.
1 mole of tyrosine
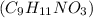 weighs = 181 g/mol
weighs = 181 g/mol
0.5 moles of tyrosine
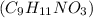 weigh
weigh
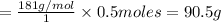
b) According to Avogadro's law, 1 mole of an ideal gas occupies 22.4 Liters at Standard conditions of temperature and pressure (STP).
1 mole of gas at STP occupy = 22.4 L
1.5 moles of gas at STP occupy =