Answer:
2KClO₃ → 2KCl + 3O₂
Step-by-step explanation:
The unbalanced expression is given as:
KClO₃ → KCl + O₂
The reaction equation above is unbalanced and we need to choose between the numbers 2,3,4 and 5 to finally the correct expression that will obey the law of conservation of mass.
aKClO₃ → bKCl + cO₂
Let a, b and c be the numbers that will balance the expression;
Conserving K;
a = b
Conserving Cl;
a = b
Conserving O;
3a = 2c
let a = 1, b = 1 and c =
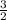 multiply through by 2;
multiply through by 2;
a = 2, b = 2 and c = 3
So, the balance expression insert;
2KClO₃ → 2KCl + 3O₂