Answer:
Positive charge.
Step-by-step explanation:
Hello!
In this case, since elements belonging to groups 1A, 2A and 3A are mostly metals, we infer they have the capacity to lose electrons and therefore they become positively charged.
Some examples may be:
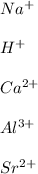
Best regards!