Answer:
68.42%
Explanation:
We need to find the percent of Cr in
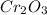 .
.
Mass of Cr = 52 amu
Mass of O = 16 amu
Firstly, we will find the molar mass of
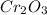 as follows :
as follows :
M = 2(52) + 3(16)
M = 152 amu
Percent of Cr in
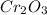 will be :
will be :
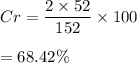
Hence, the required percentage is 68.42%.