The periodate ion is the ion that is shown in option B
What is the periodate ion?
One iodine and four oxygen atoms make up the anion known as the periodate ion, or
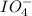 . Iodine is in the +7 oxidation state and has an overall charge of -1 in the periodate ion. The iodine atom is at the core of the tetrahedral structure of the periodate ion, surrounded by four oxygen atoms.
. Iodine is in the +7 oxidation state and has an overall charge of -1 in the periodate ion. The iodine atom is at the core of the tetrahedral structure of the periodate ion, surrounded by four oxygen atoms.
Because iodine is located in Group 17 (VIIA) of the periodic table, the prefix "period-" is the source of the chemical formula for the periodate ion.