The volume using the cylinder formula : V=πr²h
Further explanation
Charles's Law
When the gas pressure is kept constant, the gas volume is proportional to the temperature
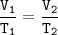
This experiment is an illustration of Charles's law, to determine the relationship between temperature and gas volume
If a certain amount of gas is heated, the volume will increase
So in this experiment, the temperature was changed as an independent variable and tube volume as a fixed variable
Because the tube is cylindrical, to find the volume using the cylinder formula
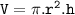
Meanwhile, to find the radius, you can use a caliper to measure the diameter of the tube and the result will be divided by 2