Given that,
The wavelength of microwave radiation = 1 cm = 0.01 m
To find,
The frequency and the energy of a single photon of this radiation.
Solution,
Let f be the frequency of the wave. The relation between frequency and wavelength is given by :
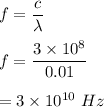
Let E be the energy of the wave. The energy of the wave is given by :
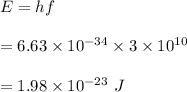
We know that,
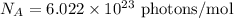
Energy :
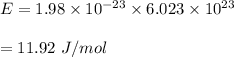
Hence, this is the required solution.