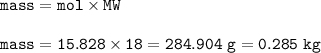The theoretical yield (in kilograms) of water produced from this reaction : 0.285 kg
Further explanation
Reaction
2C₁₂H₂₄O₁₁ + 25O₂ → 24CO₂ + 24H₂O
mass of C₁₂H₂₄O₁₁ - isomaltitol = 454 g
mol C₁₂H₂₄O₁₁(MW=344.31 g/mol) :

mol H₂O based on C₁₂H₂₄O₁₁ as limiting reactant(O₂-oxygen as an excess)
From the equation above, mol ratio of C₁₂H₂₄O₁₁ : H₂O = 2 : 24, so mol H₂O:

then mass H₂O (MW=18 g/mol) :