Answer:
sodium nitrate, because it is the solute
Step-by-step explanation:
To find the molarity of the solution, Josh should determine the number of moles of the sodium nitrate.
Molarity is defined as the number of moles of solutes in a given volume of solution.
Molarity =
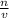
n is the number of moles of solute
v is the volume of solution