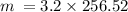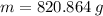Answer:
820.864 g
Step-by-step explanation:
1) The atomic mass of sulfur (found from the periodic table) is 32.065 amu. Use this mass to find the molar mass of Sulfur. Sulfur is S8 so the molar mass of sulfur is:
8 × 32.065 = 256.52 g/mol
2) To find the mass use the formula:
m = n × M where m is the mass, n is the number of moles, and M is the molar mass.
3)