Answer:
148.85 mL
Step-by-step explanation:
The reactions that take place are:
- 3HCl + Al(OH)₃ → AlCl₃ + 3H₂O
- 2HCl + Mg(OH)₂ → MgCl₂ + 2H₂O
Now we calculate how many HCl moles would react with 338 mg of Al(OH)₃. First we convert Al(OH)₃ mg into mmol, using its molar mass:
- 338 mg Al(OH)₃ ÷ 78 mg/mmol = 4.33 mmol Al(OH)₃
Then we convert mmol Al(OH)₃ into mmol HCl:
- 4.33 mmol Al(OH)₃ *
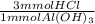 = 13 mmol HCl
= 13 mmol HCl
Now we do the same calculations for Mg(OH)₂, using its molar mass:
- 489 mg Mg(OH)₂ ÷ 58.32 mg/mmol = 8.38 mmol Mg(OH)₂
And convert into mmol HCl:
- 8.38 mmol Mg(OH)₂ *
 = 16.77 mmol HCl
= 16.77 mmol HCl
Now we add the reacting mmoles of HCl together
- 13 mmol HCl + 16.77 mmol HCl = 29.77 mmol HCl
Finally we calculate the volume of a 0.20 M solution that contains 29.77 mmoles of HCl:
- 0.20 M = 29.77 mmol HCl / V