Answer:
n = 0.288 moles
Step-by-step explanation:
Given mass = 32 g
The molar mass of CaCl₂ is:
M = 40.078 + 2(35.453)
= 110.98 g/mol
We need to find the number of moles in 32 g of CaCl₂. Let there are n no of moles.
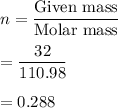
So, there are 0.288 moles in 32 g of CaCl₂